Research
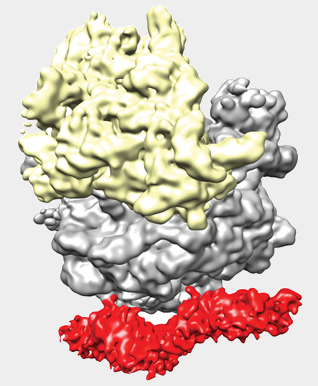
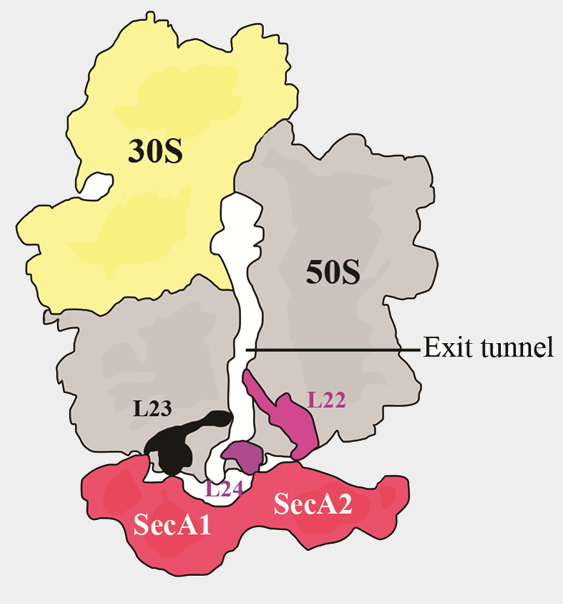
Role of SecA in protein translocation
We are interested in determining 3D structures of macromolecular protein complexes to understand their function and mechanism. We employ Cryo-Electron Microscopy with single particle reconstruction as our main tool for structural determination. Our laboratory is equipped with advanced Electron Microscopes and high speed computing clusters for routine single particle reconstruction work.
We are mainly interested in the following research areas:
- Protein translocation across cellular membranes
- Co-translational protein folding
- Protein secretion
- Translational stalling and regulation

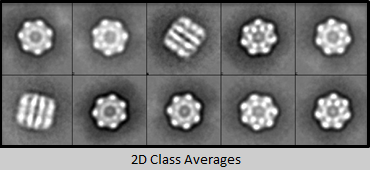
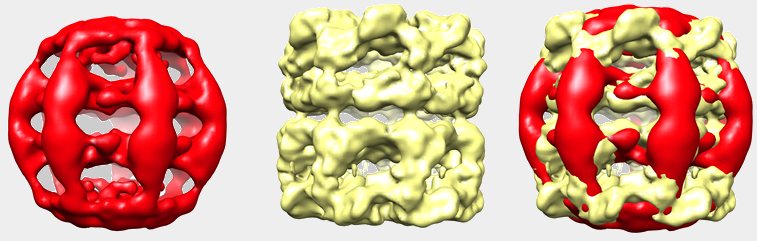
Mitochondrial Chaperonine (neg-stained)
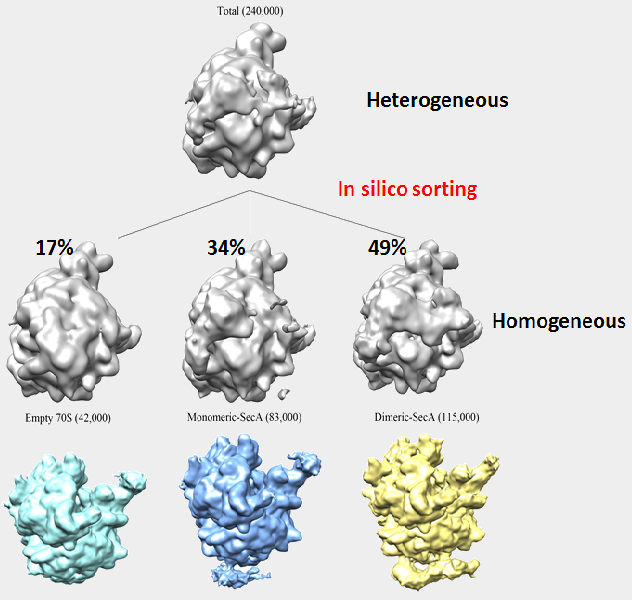
Cryo-EM and single particle reconstruction
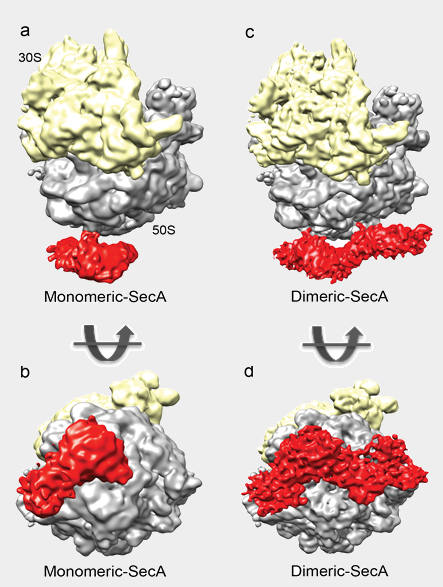
Structures of a monomeric- and dimeric- SecA bound to the ribosome (10 and 8.5 Å)
