Main group chemistry and Catalysis
Our research principally focuses on the design and development of novel p-block element-containing molecules, and their application in catalysis. The selected examples are shown below.
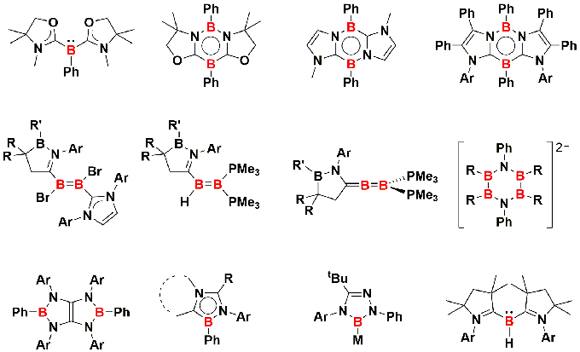
I. Isolation of highly reactive intermediate and development of unusual molecules
Typical tricoordinate organoboranes possess the boron atom in the +III oxidation state, and they usually act as Lewis acids and electrophiles. Our group has been committed to the development of organoboron species featuring a low-valent or nucleophilic boron center. Those boron compounds do not only serve as building block for the construction of novel boron-containing molecules but also activate enthalpically strong bonds under mild reaction conditions. Moreover, the Lewis basic boron compound may act as a signma-donor ligand of transition metal complexes, one of which exhibits the catalytic activity to promote the formation of C-C, C-N, and C-O bonds.
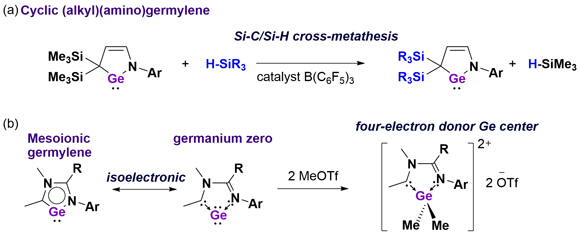
II. New Germylene family
Germylenes bearing a Ge atom in the +2 oxidation exhibit various chemical behaviours including coordination to metals and cycloaddition reactions. By fine-tuning of the structural and electronic nature, we have developed a highly reactive germylene. Interestingly, we discovered that a catalytic amount of B(C6F5)3 promotes a Si-C/Si-H cross-metathesis reaction between cyclic (alkyl)(amino)germylenes and various hydrosilanes (R3SiH), which has allowed straightforwardly accessing to new germylenes (Fig a). Furthermore, we newly synthesized a mesoionic germylene species bearing a Ge atom in the zero oxidation state (Fig b). Thus, the germanium center in this molecule formally possesses two lone pairs, which serves as a four-electron donor.
III. Metal-free catalysis with main group molecules
Development of catalysts constructed by earth-abundant cheap elements instead of precious metals presents a significantly economical approach because it eliminates the major drawbacks of the metal catalysis that are the excessive cost of metal complexes (= metal + ligands) and in many cases the toxicity of the metals. Recently, we have demonstrated that the phosphorus compound could serve as efficient catalysts for several industrially important catalyses.
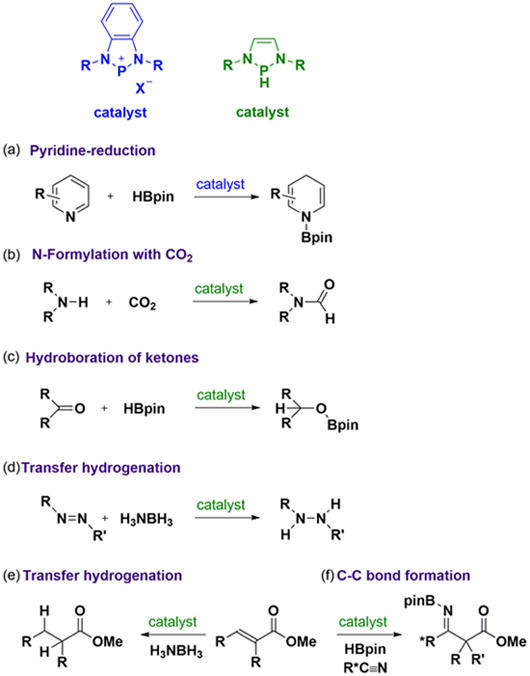
(a) Pyridine reduction Dihydropyridine (DHP) derivatives are ubiquitous in naturally occurring molecules, biologically active agents such as NADH, and pharmaceutically important molecules such as Hantzsch esters. Hence, the development of efficient methodologies for the construction of a DHP skeleton has attracted considerable interest in synthetic chemistry. N-heterocyclic phosphenium triflates (NHPOTf) serve as efficient catalysts for the regio- and chemoselective hydroboration of pyridines under an ambient condition with good functional group tolerance, producing a variety of DHP derivatives (Fig a).
(b) N-Formylation with CO2 Carbon dioxide is plentiful, inexpensive, and non-toxic. Hence, the development of a methodology to utilize carbon dioxide as a C1 building block for the production of functionalized chemicals has attracted attention in petrochemistry and synthetic chemistry. We have developed a catalytic transformation of amines with CO2 into formamides using the diazaphospholene catalyst (Fig b). This system will provide a general methodology for various CO2 functionalizations, and contribute to the field of green chemistry.
(c) Hydroboration of ketones Carbonyl reductions are useful methodologies for the synthesis of various products, and therefore have been extensively used in organic syntheses and industrial processes. Among them, hydroboration has attracted considerable attention, since boron hydrides are relatively stable and can easily be stored, which evades the defects of the use of highly flammable and highly pressurized hydrogen gas. We have shown the first example of metal-free catalytic hydroboration using the diazaphospholene (Fig c).
(d) Transfer hydrogenation The use of ammonia-borane as a hydrogen source for hydrogenation reaction is considered to be superior to the use of hydrogen gas which is highly combustible and therefore difficult to be stored and transported safely as well as economically. By employing a diazaphospholene as the catalyst, transfer hydrogenation of N=N double bond (Fig d) and ???-unsaturated esters (Fig e) can be achieved with ammonia-borane as the hydrogen source.
(e) C-C bond formation The C-C bond-formation reaction is one of the most significant protocols in organic chemistry, and ubiquitous in synthesizing diverse molecules, ranging from natural products, pharmaceuticals, polymers, to materials. We have demonstrated that the formation of boryl enolate intermediate via 1,4-hydroboration of aryl substituted α,β-unsaturated esters catalyzed by the diazaphospholene can be followed by the further reaction with acetonitrile to form amino-diester products through a C-C coupling reaction (Fig f).

I. Isolation of highly reactive intermediate and development of unusual molecules
Typical tricoordinate organoboranes possess the boron atom in the +III oxidation state, and they usually act as Lewis acids and electrophiles. Our group has been committed to the development of organoboron species featuring a low-valent or nucleophilic boron center. Those boron compounds do not only serve as building block for the construction of novel boron-containing molecules but also activate enthalpically strong bonds under mild reaction conditions. Moreover, the Lewis basic boron compound may act as a signma-donor ligand of transition metal complexes, one of which exhibits the catalytic activity to promote the formation of C-C, C-N, and C-O bonds.

II. New Germylene family
Germylenes bearing a Ge atom in the +2 oxidation exhibit various chemical behaviours including coordination to metals and cycloaddition reactions. By fine-tuning of the structural and electronic nature, we have developed a highly reactive germylene. Interestingly, we discovered that a catalytic amount of B(C6F5)3 promotes a Si-C/Si-H cross-metathesis reaction between cyclic (alkyl)(amino)germylenes and various hydrosilanes (R3SiH), which has allowed straightforwardly accessing to new germylenes (Fig a). Furthermore, we newly synthesized a mesoionic germylene species bearing a Ge atom in the zero oxidation state (Fig b). Thus, the germanium center in this molecule formally possesses two lone pairs, which serves as a four-electron donor.

III. Metal-free catalysis with main group molecules
Development of catalysts constructed by earth-abundant cheap elements instead of precious metals presents a significantly economical approach because it eliminates the major drawbacks of the metal catalysis that are the excessive cost of metal complexes (= metal + ligands) and in many cases the toxicity of the metals. Recently, we have demonstrated that the phosphorus compound could serve as efficient catalysts for several industrially important catalyses.

(a) Pyridine reduction Dihydropyridine (DHP) derivatives are ubiquitous in naturally occurring molecules, biologically active agents such as NADH, and pharmaceutically important molecules such as Hantzsch esters. Hence, the development of efficient methodologies for the construction of a DHP skeleton has attracted considerable interest in synthetic chemistry. N-heterocyclic phosphenium triflates (NHPOTf) serve as efficient catalysts for the regio- and chemoselective hydroboration of pyridines under an ambient condition with good functional group tolerance, producing a variety of DHP derivatives (Fig a).
(b) N-Formylation with CO2 Carbon dioxide is plentiful, inexpensive, and non-toxic. Hence, the development of a methodology to utilize carbon dioxide as a C1 building block for the production of functionalized chemicals has attracted attention in petrochemistry and synthetic chemistry. We have developed a catalytic transformation of amines with CO2 into formamides using the diazaphospholene catalyst (Fig b). This system will provide a general methodology for various CO2 functionalizations, and contribute to the field of green chemistry.
(c) Hydroboration of ketones Carbonyl reductions are useful methodologies for the synthesis of various products, and therefore have been extensively used in organic syntheses and industrial processes. Among them, hydroboration has attracted considerable attention, since boron hydrides are relatively stable and can easily be stored, which evades the defects of the use of highly flammable and highly pressurized hydrogen gas. We have shown the first example of metal-free catalytic hydroboration using the diazaphospholene (Fig c).
(d) Transfer hydrogenation The use of ammonia-borane as a hydrogen source for hydrogenation reaction is considered to be superior to the use of hydrogen gas which is highly combustible and therefore difficult to be stored and transported safely as well as economically. By employing a diazaphospholene as the catalyst, transfer hydrogenation of N=N double bond (Fig d) and ???-unsaturated esters (Fig e) can be achieved with ammonia-borane as the hydrogen source.
(e) C-C bond formation The C-C bond-formation reaction is one of the most significant protocols in organic chemistry, and ubiquitous in synthesizing diverse molecules, ranging from natural products, pharmaceuticals, polymers, to materials. We have demonstrated that the formation of boryl enolate intermediate via 1,4-hydroboration of aryl substituted α,β-unsaturated esters catalyzed by the diazaphospholene can be followed by the further reaction with acetonitrile to form amino-diester products through a C-C coupling reaction (Fig f).
2011 Kinjo Laboratory. All Rights Reserved.