News
2017.08 One postdoctoral position is available from March 2018
2017.04 Congratulations to Luthfi for his paper “Reactivity of a Base-Stabilized Germanium(I) Dimer toward Group 9 Metal(I) Chloride and Dimanganese Decacarbonyl” has been accepted by Inorganic Chemistry
2017.04 Group lunch at Pen & Inc to celebrate Samuel’s graduation, welcome Jiajia to join our group and Sabrina’s birthday
2107.03 Congratulations to Dr Li Yan for his paper “Diverse Bonding Activations in the Reactivity of a Pentaphenylborole toward Sodium Phosphaethynolate: Heterocycle Synthesis and Mechanistic Studies” has been accepted by Inorganic Chemistry
2017.02 Congratulations to Yuliang and Bi Xiang for their paper “Reactivity of an Amidinato Silylene and Germylene toward Germanium(II), Tin(II) and Lead(II) Halides” has been accepted by Dalton Trans
2017.01 Group lunch to celebrate Chinese New Year at Hotel Jen Tanglin
2017.01 Congratulations to Yuliang for his paper “Donor-Acceptor Stabilized Tetra(silanimine)” has been accepted by Inorganic Chemistry
2017.01 Congratulations to Shuhua for her paper “Delocalized Hypervalent Silyl Radical Supported by Amidinate and Imino Substituents” has been accepted by Inorganic Chemistry
RESEARCH INTEREST
My research group investigates the chemistry of very low oxidation state and coordination number p- and d-block complexes with emphasis on their unprecedented electronic structures and reactivities, which were thought to be inexistent and unstable at room temperature, leading to possible application in catalysis, materials chemistry, hydrogen storage, etc.. Examples of specific areas of research currently being investigated can be found below:
1. Designing strong π-accepting ligands for the stabilization of green, abundant, low coordinate main-group(0) and transition metal(0) complexes
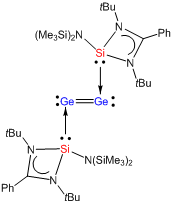
A catalytic cycle is usually initiated by the coordination of an organic substrate to the metal centre in a catalyst, followed by further chemical processes. Thus, the key in successful catalytic transformation is to generate highly Lewis acidic and catalytically active metal centres, so that they can easily bind with a nucleophilic organic substrate to initiate the catalytic process. My group is developing neutral and strong π-accepting ligands comprising a vacant orbital, which facilitates the π-back bonding from the filled orbitals on the main-group and transition metal centre to the ligand, leading to enhance the Lewis acidity of the metal centre in a catalyst. For example, the N-heterocyclic silylene LSiN(SiMe3)2 (L = chelating amide) can function as a π-accepting ligand for the stabilization of a novel digermanium(0) moiety (Angew. Chem. Int. Ed., DOI: 10.1002/anie.201408347).
 |
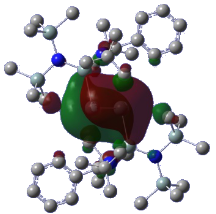 |
| NHSi-stabilized digermanium(0) complex | HOMO showing the π-back bonding from the digermanium(0) to the low valent silicon centre |
2. Heavier main-group elements-containing -conjugated systems
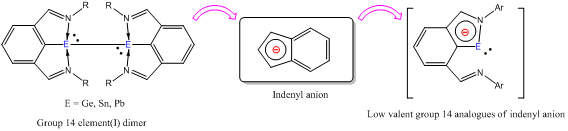
Main-group elements contain a variety of properties in terms of electronegativity, Lewis acidity/basicity, and valency/coordination numbers that cannot be found in carbon atom. The incorporation of a main-group element such as B, Si, P, S, and Se, into a -conjugated framework intrigues unique electronic and photophysical properties that are difficult to attain in the conventional carbon-based -electron materials. In the past three years, my group heavily involved in this area and has developed some novel strategy to utilize low valent group 14 element(I) dimer to construct a variety of -conjugated systems, which show extraordinary electronic structures that cannot be observed in carbon congeners. These include the aromatic plumbylidenide anion and germylidendiide dianion radical, which illustrate the 2p orbitals of C/N atoms and np orbitals (n = 3-6) of heavier group 14 elements can be sufficiently overlapped to form aromatic compounds (Angew. Chem. Int. Ed. 2013, 52, 6298; Angew. Chem. Int. Ed. 2014, 53, 8455; Chem. Eur. J. 2010, 16, 12956). The incorporation of heavier group 14 elements into 4n -conjugated systems lead to perturb the antiaromaticity of -conjugated systems and the resulting compounds show extensive electron delocalization (Angew. Chem. Int. Ed. 2013, 52, 12346; Chem. Eur. J. 2013, 19, 14726; Chem. Eur. J. 2012, 18, 2685; Chem. Eur. J. 2012, 18, 4258).
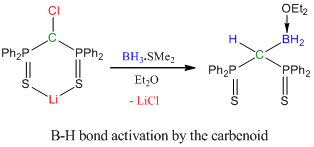
3. The “transition metal-like” reactivity of main-group compounds
The most abundant elements in the earth’s crust are main-group elements (oxygen: 46.6%, silicon: 27.7%, aluminium 8.1%, calcium 3.6%, sodium 2.8%, potassium 2.6% and magnesium 2.1% of the earth’s crust). They are less toxic and cheaper than transition metals. As a result, utilizing these elements in performing activation of small molecules or enthalpically strong bonds would be a sustainable option, which is tremendously beneficial not only for scientific community, but also for our society in general by saving money and resources. In the past two years, my group modified main-group compounds, which possess electronic properties and coordinative flexibility similar to those on transition metal complexes, for example, the activation of BH3 by a stable highly electrophilic carbenoid complex (J. Am. Chem. Soc. 2013, 135, 8774; Organometallics 2013, 32, 498). Such activation is never observed in transition metal complexes.