Major Research Activities
Roles of FKBPs in malaria and conformational disorders
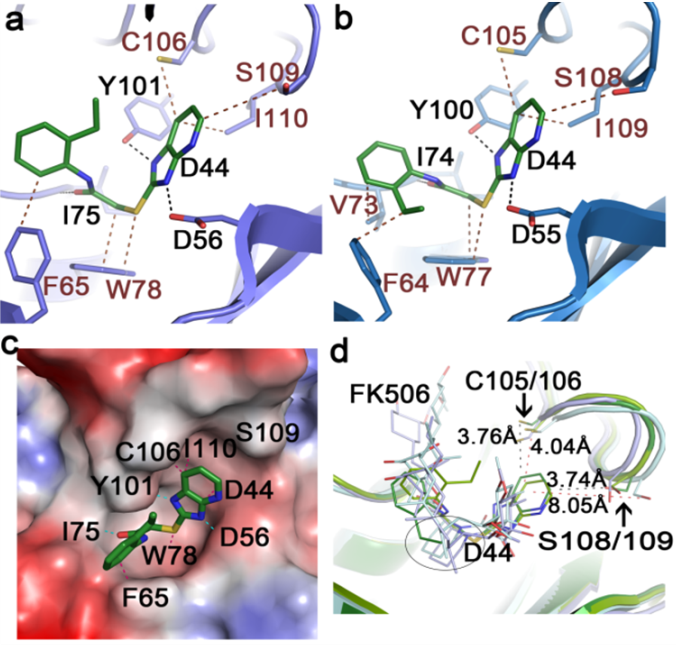
We’ve been studying molecular and structural characteristics of several FKBP family proteins from human malaria parasites Plasmodium. FK506 exerts an antimalarial activity. The mechanism of the drug action involves the molecular interaction with target proteins PfFKBP35 and PvFKBP35, which are only FKBP members from P. falciparum and P. vivax, respectively. These tripartite FKBP family members play important roles in the life cycle of the parasites. We have determined the structures of the FK506 binding domain (FKBD) of P. falciparum and P. vivax PfFKBP35 and PvFKBP35 in apo form and in complex with FK506 and also substrate, providing insights into possible routes for rational drug design targeting the parasite immunophilins. Our structure-based drug design efforts resulted in a small molecule FKBP inhibitor (SRA) that exhibits a potent antimalarial activity. We obtained a high resolution crystallographic structure of FKPB35 in complex with SRA in which SRA occupies the FK506-binding pocket, but shows neither calcineurin inhibition nor immunosuppressive activity. Currently we are evaluating SRA profile in a malaria animal model system.
We’ve been also studying the roles of FKBPs in neuronal cells with an emphasis on the cellular mechanisms responsible for their neuroprotective and neurotrophic activities. Protein misfolding has been implicated in the pathophysiology of neurodegenerative diseases. The unmet need for therapeutic intervention in such conformational diseases continues to drive the search for target molecules. As FKBPs have emerged as potential cellular target for treatment of Parkinson’s disease and other neurological disorders, non-immunosuppressive neuroimmunophilin ligands (NILs) with their small size, target specificity, bioavailability and stability provide excellent scaffolds for the development of new drugs. Although our present knowledge of the mode of action of NILs is still fragmentary, there is increasing evidence that the neurotrophic, neuroprotective and restorative potential of these compounds is mediated by signaling pathways that can have antagonistic or additive effect. Much work needs to be done so as to identify unique neuroimmunophilins and their interacting partners, assess their cellular function as well as their response to injury. Furthermore, issues such as reproducibility of preclinical data, structure-activity relationship studies, drug evaluation in appropriate animal models, and implementation of proper clinical designs and endpoints needs immediate attention as such information will aid in the development of novel NILs with improved efficacies, target selectivity and potency. FKBPs and NILs seem to be a promising area for therapeutic intervention of Parkinson’s disease and other neurodegenerative disorders.
Regulation of Bcl-2/Bcl-XL
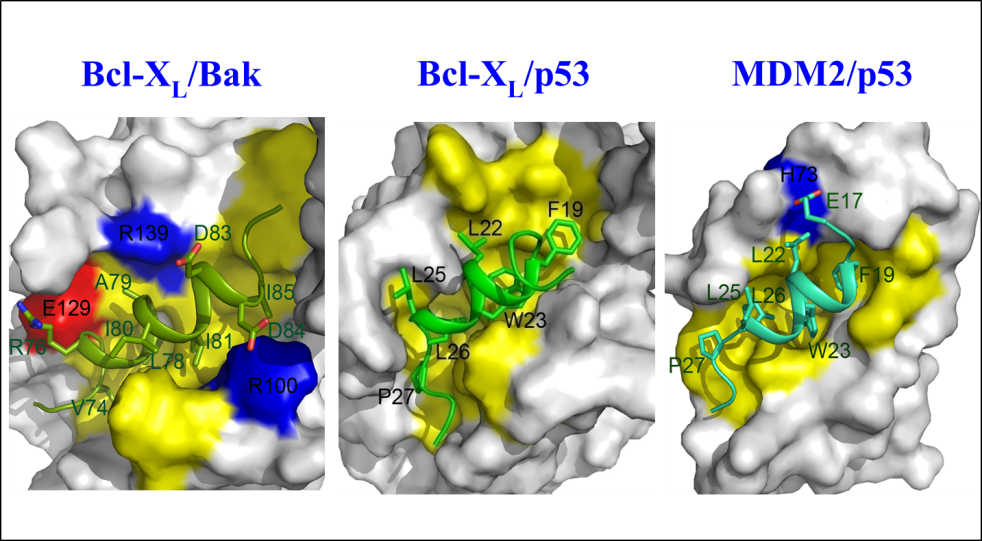
As cancer is attributable to the homeostatic imbalance between cell growth and cell death, dysregulation of apoptosis is closely linked to the development of cancer. The aberrant expression of Bcl-2, which is one of key players in apoptosis, has been implicated in a number of diseases including cancer and autoimmunity and neurodegenerative disorders. In particular its overexpression is linked to resistance to conventional chemotherapy. Thus, Bcl-2 represents a good target for the treatment of cancers, especially those in which Bcl-2 is overexpressed and for which the conventional chemotherapy has failed. The structure of Bcl-XL and Bcl-XL-BAK BH3 peptide revealed several key features in the ligand-binding site and molecular basis of heterodimerization mechanism between anti-apoptotic and pro-apoptotic proteins and provided a platform to aid design drugs to inhibit protein-protein interaction between pro-and anti-apoptotic Bcl-2 family proteins. While the classical regulatory mechanism of Bcl-2 family proteins through the heterodimer formation between the anti-apoptotic and pro-apoptotic Bcl-2 family proteins has been extensively studied, the recently emerging alternative regulatory mechanisms of Bcl-2 family remain relatively unknown and thus deserve attention due to potential functional implications in apoptosis as the Bcl-2 family proteins also interact with non-canonical binding partners such as p53 and FKBP38. To this end, we demonstrated that the N-terminal domain of p53, in particular, the MDM-2 binding region is involved in the molecular interaction with Bcl-2 family proteins. The results provide insights into pleiotypic role of p53 and its transcription-independent mechanism in apoptosis. We also showed in collaboration with Dr SW Chi’s group that Nutlin-3, a potent MDM2 antagonist binds to the BH3 binding pocket in Bcl-XL. The emerging roles of the Bcl-2 family certainly support the hypothesis that Bcl-2 and its related family are possibly involved in several cellular pathways and their versatile biological roles are well orchestrated in response to different stimuli perturbing cellular homeostasis.
Bcl-2 and Bcl-XL are localized at the mitochondrial membrane and function as membrane proteins during apoptosis. Although channel or pore-forming activity of Bcl-2 family have been demonstrated by biochemical and biophysical approaches, currently we still don’t fully understand how the anti-or pro-apoptotic Bcl-2 family execute apoptosis or resist cell death in membrane environment. This is in part due to a lack of three-dimensional structural information of Bcl-2 family proteins in membrane phase. The Bcl-2 research community still waits for the membrane topology of Bcl-2 family proteins. The detailed 3D structural portraits will provide pivotal information about their molecular mechanisms in apoptosis. To this end, we are keen to determine three-dimensional structure of Bcl-2/Bcl-xL in a membrane-mimicking environment. Over the years, we’ve been making steady progress toward this goal. The expected outcomes might provide valuable information on how Bcl-2 family proteins modulate their anti-and pro-apoptotic activities in membrane environment in response to apoptotic stimuli. Moreover the reconstituted Bcl-2 family proteins in membrane might be a good tool for evaluating inhibitors against membrane-associated Bcl-2 family members.
Protein-phospholipid interaction
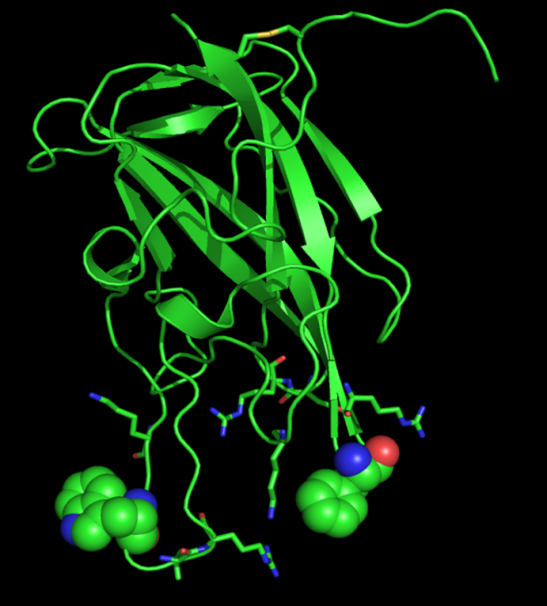
Protein-phospholipid interactions are keys to cellular signal transduction pathways, which mobilize adaptors and second messengers by recognizing membrane components and targeting their host proteins to cellular membranes. We determined the first Pleckstrin Homology (PH) domain structure and demonstrated that PH domains specifically bind to phosphoinositides such as PIP2. In recent years, we’ve been studying the molecular basis of interaction between phosphatidylserine (PS) and its interacting protein in response to apoptotic stimuli. “Eat-Me” signals are presented on the surface of apoptotic cells and phagocytes are recruited to apoptotic cells and engulf them. MFG-E8, one of secreted proteins from macrophages, functions as a bridging molecule between macrophages and apoptotic bodies. MFG-E8-C2 domain plays an important role in recognizing phosphatidylserine (PS), which is one of the “Eat-Me” signals exposed on dying cells. We study the molecular basis of the interaction between MFG-E8 and PS. We have determined NMR solution structure of MFG-E8-C2 domain and characterized its mode of interaction with phosphatidylserine in response to apoptotic stimuli. As PS is exposed on surface of apoptotic bodies and serves as biomarker for the detection of apoptosis, one of our research directions is to develop fluorescent C1-C2 or MFG-E8 as a PS-detection system during apoptosis. The outcomes of the project might shed light on the proposed role of MFG-E8 in autoimmune disorder. In collaboration with Dr Chengyu Liang (Univ. of Southern California), we’ve been also studying the molecular interaction between PI(3)P and the C2 domain of UVRAG, a Beclin1-binding autophagic factor. These findings identify a regulatory mechanism that coordinates Golgi-ER retrograde and autophagy-related vesicular trafficking events through physical and functional interactions between UVRAG, phosphoinositide and their regulatory factors, thereby ensuring spatiotemporal fidelity of membrane trafficking.
Molecular mechanism of epigenetic regulation of histone through phosphorylation by Vaccinia-related kinase 1
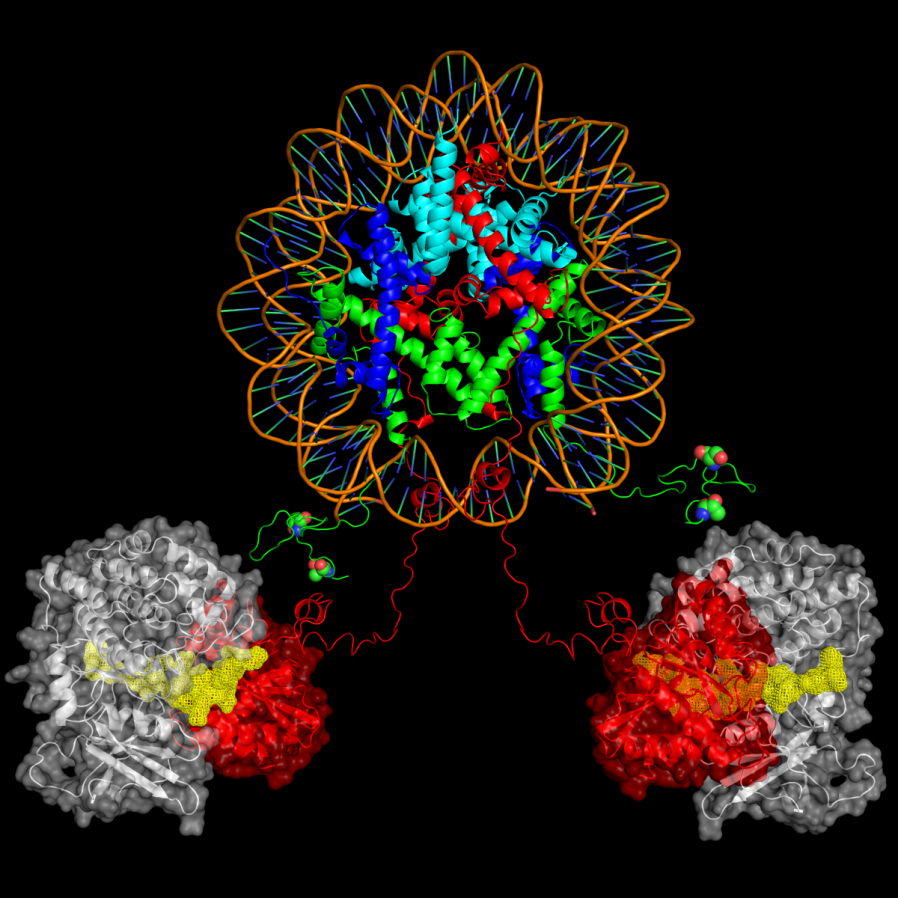
Vaccinia-related kinase 1 (VRK1) is one of the mitotic kinases which play important roles in cell cycle, nuclear condensation and transcription regulation. We have determined NMR solution structure of a catalytically active form of human VRK1 with its extended C-terminal tail (residues 1-361). To our knowledge, VRK1 NMR structure is the first reported protein kinase structure ever determined by NMR spectroscopy. The NMR solution structure of human VRK1 reveals that the C-terminal tail orients toward the catalytic site and forms a number of interactions that are critical for structural stability and catalysis. In addition to the conventional interactions, some of the residues present on the extended C-terminal tail also interact with the ligand. These observations also substantiate the role of the extended C-terminal tail in the biological activity of VRK1. And then we showed in collaboration with Prof KT Kim that a histone variant macro domain-containing histone H2A1.2 functions as a suppressor against VRK1 during interphase. The level of macroH2A1.2 was markedly reduced in mitotic phase and the macroH2A1.2-mediated inhibition of histone H3 phosphorylation occurred mainly during interphase. We also found direct interaction and binding natures between VRK1 and macroH2A1 by NMR spectroscopy. Hence our findings provide valuable insight into the underlying molecular mechanism about an epigenetic regulation of histone H3 during cell cycle.
As the three-dimensional structure of VRK1 is available with us and histone variant mH2A1.1-mediated inhibition of the mitotic kinase VRK1 activity and its functional significance on the histone H3 phosphorylation has been newly discovered, structural basis of VRK1 in complex with it substrates and the elucidation of the novel inhibitory role of the macro histone on VRK1 activity might provide insights into the regulatory mechanism of the mitotic histone kinase during cell cycle and contribute to our understanding on the dynamic epigenetic control of histone through phosphorylation. Thus, in collaboration with Prof Kyong-Tai Kim (POSTCH), we wish to reconstitute nucleosome core particle (NCP) or chromatin array using the variant histone and study the regulatory role of mH2A1 on VRK1 activity and the functional significance in terms of mH2A1 oscillation and dynamics of histone phosphorylation and biophysical characteristics of the hybrid NCP by NMR. As aberrant expression is associated with cancers such as squamous cell carcinomas of the head and neck, efforts will be made to discover small inhibitors targeting human VRK1.